
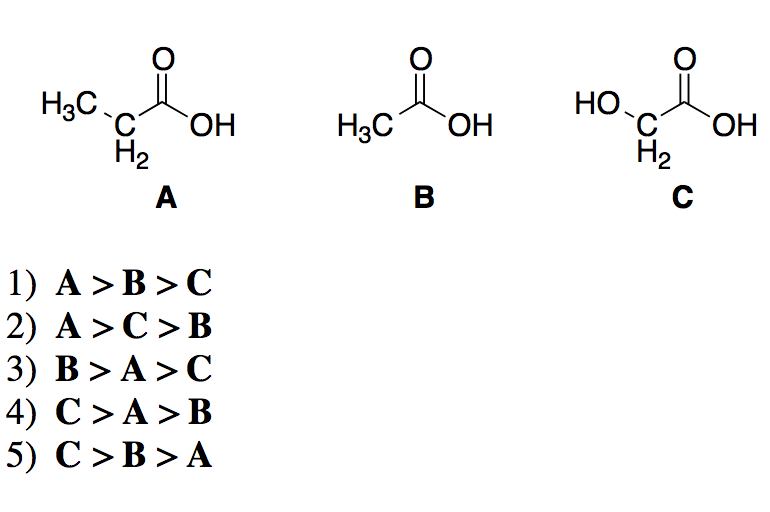
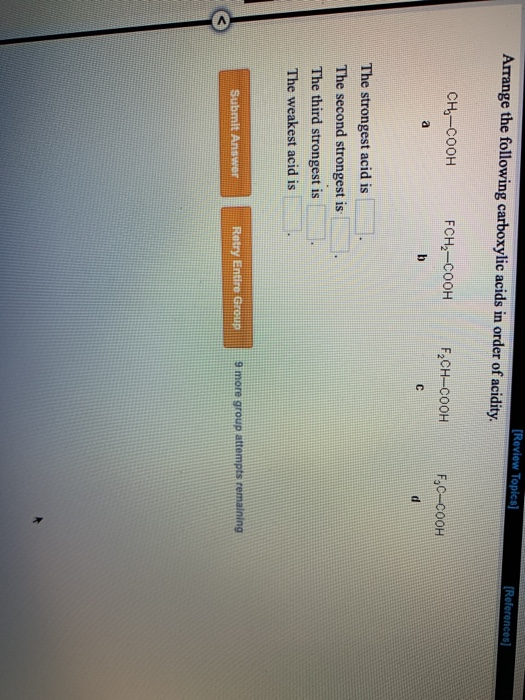
Therefore it increases electron density as a result removal of proton becomes difficidt. Increasing order of their acidic strength: 2 > 3 > 1. Incase of acetic acid, methyl group is electron doner. Chlorine is electronegative element, it attracts the electrons towards itself, as a result removal of proton from chloroacetic acid becomes easier. If you have any queries, drop a comment below and we will get back to you. Acetic acid < Fromic acid< Chloroacetic acidProton donors are acids. We hope this article on ‘Acidity of Carboxylic Acids’ has helped you. Arrange the following carboxylic acids in order of acidity.ClCOOHCOOHCOOHCOOHThe strongest acid is The second strongest is The third strongest is The weakest. Carboxylic Acid Questions 1. Benzoic acid, 4-Nitrobenzoic acid, 3, 4. Which is more acidic, alcohol or phenol?Īns: Phenol is more acidic than alcohol since phenoxide ion is more stabilized than alkoxide ion due to the resonance effect. View Carboxylic Acid Questions-1.doc from CO 2H at Harvard University. Q.9 Arrange the following compounds in increasing order of their acid strength.
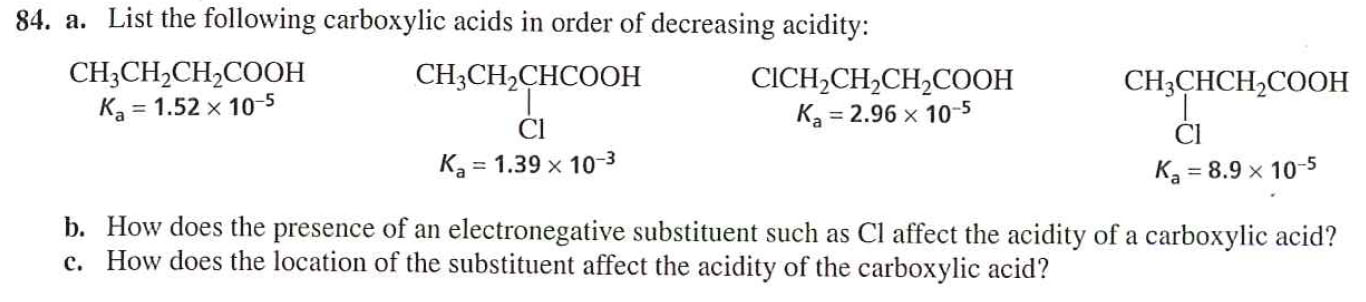
Therefore, carboxylic acids are stronger acids than alcohol. However, in alcohols, no resonance is possible for the alcohols as well as alkoxide ions. (B) Butanoic acid, propanoic acid, ethanoic acid, methanoic acid. The ranking of these acids in order of increasing acidity is: CH3CH2COOH


 0 kommentar(er)
0 kommentar(er)
